Change of State and Latent Energy
During a phase change, the temperature of a substance does not increase or decrease. This is because the thermal energy during a phase change goes into breaking the intermolecular bonds between particles rather than increasing the kinetic energy of the particles. The latent heat is the energy released or absorbed during a change of state. ‘latent’ means hidden or unseen.
The latent heat is calculated using the formula:
![]()
where:
![]() = the heat energy transferred in Joules (J)
= the heat energy transferred in Joules (J)
![]() = mass (kg)
= mass (kg)
![]() = the latent heat (Jkg−1)
= the latent heat (Jkg−1)
The value of L is dependent on the substance being considered and also the phase change. It takes a lot more energy to change a liquid to a gas than it does a solid to a liquid. This means the latent heat, L, for water to steam will be higher than for ice to water.
When considering a phase change from solid to liquid (melting), ![]() is described as the latent heat of fusion (
is described as the latent heat of fusion (![]() ). When considering a phase change from liquid to a gas (boiling),
). When considering a phase change from liquid to a gas (boiling), ![]() is described as the latent heat of vaporisation (
is described as the latent heat of vaporisation (![]() ).
).
Consider water:
- Latent heat of fusion (
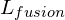 ) = 3.34×105 Jkg−1
) = 3.34×105 Jkg−1 - Latent heat of vaporisation (
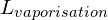 ) = 22.5×105 Jkg−1
) = 22.5×105 Jkg−1
Example 1:
How much energy is required to melt a 5kg block of ice?
Answer:
using: ![]()
where:
![]() = 5 kg
= 5 kg
![]() = 3.34×105 Jkg−1
= 3.34×105 Jkg−1
![]()
![]()
![]()
